In this project, we are interested in exploring non-genetic interactions which we hypothesise are an important determinant for the development and progression of vascular complications in individuals with diabetes.
Vascular complications remain the major cause of mortality and morbidity in diabetes with increasing evidence that prior glycemic exposure is a major determinant of susceptibility and progression of these disorders. Most individuals with diabetes have good health outcomes. However, many others do not. Despite the availability of effective therapies, diabetes remains the leading cause of cardiovascular disease (CVD), amputation, renal impairment and vision loss in adults. It is not simply poor metabolic or blood pressure control, as even with intensive intervention and dedicated compliance, complications still occur. Furthermore, it is not simply having the wrong genes, as genome-wide association studies have demonstrated that the genetic code explains only a fraction of the missing heritability. The most likely explanation is that there is a complex interaction between the cellular environment and genes.
Theme 1: DNA methylation projection predicts the risk of diabetic kidney disease (diabetic nephropathy)
Collaborative diabetes centres

Diabetic kidney disease methylation trajectories
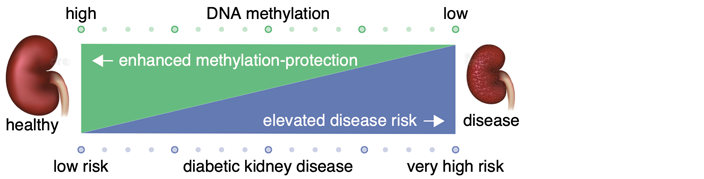
In adults with diabetes the loss of DNA methylation in several disease-related genes appears to be associated with the loss of protection and more aggressive development of diabetic kidney disease (DKD) and a poorer chance of survival.
Our studies take an unprecedented look at methylation testing of disease genes in national and international diabetes cohorts, offering important clues that reduced DNA methylation is closely associated with the increased risk of DKD. The multi-national study led by Professor Sam El-Osta of the Human Epigenetics team using methylation sequencing technology identified predictive genes associated with the development and progression of diabetic kidney disease. These discoveries will influence how we screen patients with diabetes and improve risk stratification, disease prediction and diagnosis.
These studies are important because standard assays used in the clinic rely on assessing kidney function and the level of damage to the kidney caused by diabetes. While these assays have been the mainstay of detecting progressed diabetic kidney disease, the early stages of the disease are typically without symptoms. This project will examine the development of reliable methods that improve the predictive risk and diagnostic accuracy of future progressors of DKD.
There are more than 500 million adults living with diabetes and approximately 1 in 4 adults will develop kidney disease. Diabetic kidney disease or DKD is the leading cause of chronic kidney disease and reflects the increasing prevalence of diabetes worldwide. Kidney disease is far more common in people with diabetes than in people without the condition. More than 80 per cent of cases of end-stage renal disease are caused by diabetes which is also a risk for hypertension. The total number of people living with diabetes is projected to rise to 643 million by 2030 and 783 million by 2045.
Despite the tremendous advances in genetic testing, no risk genes for diabetic kidney disease have been identified. The Human Epigenetics team using innovative sequencing techniques will develop predictive gene methylation risk scores that are tightly associated with early detection and the development of diabetic kidney disease. The researchers of the Human Epigenetics team hope that routine gene methylation testing for diabetic complications such as kidney disease will soon be a standard part of the treatment plan, as it is for more common cancers. As far as technological advancements in methods are concerned, epigenetic testing is going to be the new standard for early detection and DKD care. Renal biopsies are difficult to procure, and the novel blood-based test means the test can be readily available and used in remote areas with the added advantage of being more stable than methods measuring other biological indices.”